A new, investigational drug may be able to help individuals who suffer with Alzheimer’s disease. It does so by reducing the amyloid beta plaque in the brains of Alzheimer’s patients. Amyloid beta plaques are tangled clumps of proteins that form in the brain.
The drug works by boosting the immune system to recognise the plaque and clear it.
“We believe that’s a hint of efficacy,” study co-author Dr. Alfred Sandrock, a neurologist and an executive vice president at Biogen, said during a news briefing. “We believe that needs to be confirmed with further studies.” Biogen is the Cambridge, Massachusetts, company that funded the trial and applied to patent the drug.
The study is still ongoing and it has not yet been done to the extent where it could show if it had an effect on the symptoms of those with Alzheimer’s disease. The drug may also cause fluid accumulations in some individuals who are genetically susceptible.
Identifying the Cause of Dementia
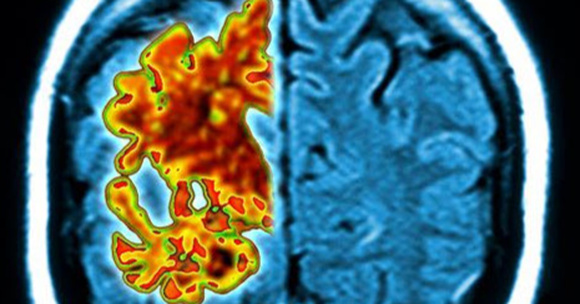
Dementia may be caused by a number of issues, but Alzheimer’s is the leading cause. More than 5 million people in the United States are affected, and many more are affected by it around the world. One of the primary things that is seen in the disease is the development of abnormal protein, called amyloid beta. When seen on brain scans, it looks like clumps of tangled fiber. The effects that it has on brain cells are responsible for many symptoms of Alzheimer’s, including mood changes, loss of function and memory loss.
Although there is not a cure for Alzheimer’s, a few treatments have had modest benefits.
Going after Amyloid Beta
Sandrock and his colleagues turned to people who do not have Alzheimer’s or dementia. By collecting chemicals from healthy older individuals as well as those who have cognitive decline, it helped them to identify some of the problem. They identified an immune compound and then created a drug that works the same in the human body. In animal studies, it seemed a target amyloid beta and helped with the effects it has on the brain.
165 patients were given infusions of either this drug, known as aducanumab, or a placebo. Then a series of brain scans were performed. The individuals who are taking aducanumab showed a decrease in the amount of amyloid beta in the brain. When they received a higher dose, the effects were even greater.
“After one year, you can see no red on the image, meaning the amyloid has almost completely disappeared,” said Dr. Roger Nitsch, a co-author of the study and the director of the Institute for Regenerative Medicine at the University of Zurich. He is also a founder of the biopharmaceutical company Neuroimmune.
Individuals who took the medication with Alzheimer’s who also had a genetic change called the APOE gene variant were more likely to experience a dangerous side effect known as amyloid-related imaging abnormalities, or ARIA. It showed up as fluid pockets in the brain.
The majority of people who developed ARIA were asymptomatic but a few individuals said they had headaches. In some cases, ARIA may increase the risk for cerebral hemorrhage and stroke.
The Results Are Unclear
The study was not done to see if the medication can produce cognitive benefit but they did find hints of cognitive benefit through their studies.
“We’re encouraged that, there appeared to be a slowing of cognitive decline at a dose-dependent manner, and also a dose-dependent slowing in functional decline,” said study co-author Dr. Stephen Salloway, a neurologist at Butler Hospital in Providence, Rhode Island.
In order to determine if the benefit is real, more studies need to be completed.
The question is if clearing amyloid beta will reduce cognitive decline. According to some researchers, amyloid beta may be a byproduct of the destructive brain process and not the cause.
Larger trials of the drug are likely to be performed and if they show improvement in the cognitive function of patients, it will help to settle the debate.
“It would be prudent to withhold judgment about aducanumab’s cognitive benefit until results from the larger trials are in,” Reiman wrote in the article.
It may also be possible that people who show symptoms have already been accumulating plaque for 15 years. By that time, much of the cognitive damage may have already occurred.
Eventually, this medication or one similar to it could be most effective when used during the first signs of plaque accumulation but when cognitive symptoms have not yet appeared.
“I still think that treating early is going to be the key,” Sandrock said.
Via: Huffington Post
Be sure to share this with your friends on Facebook
